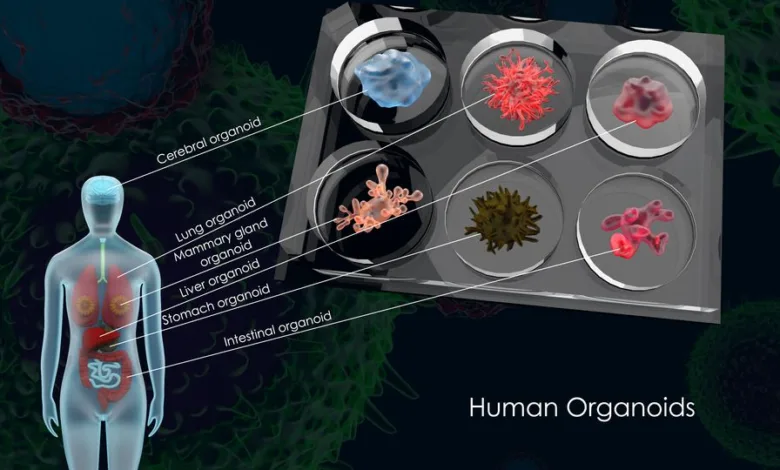

Pioneering Organoid Models Shine Light on Hopeful Paths in Pancreatic Cancer Treatment
Pancreatic cancer, particularly pancreatic ductal adenocarcinoma (PDAC), remains a formidable challenge in the realm of oncology because of its high death rate and resistance to conventional treatments. However, recent breakthroughs using organoid-based drug screening systems are shedding light on novel therapeutic approaches.
In a groundbreaking study led by researchers at Weill Cornell Medicine, a unique drug screening platform employing lab-grown tissues called organoids proved instrumental in identifying a potential game-changer for future pancreatic cancer treatments. Published in Cell Stem Cell on December 26, the study focused on testing analysis on pancreatic tumor organoids over 6,000 compounds, carrying a prevalent cancer-driving mutation.
The standout discovery was the efficacy of an existing heart drug, perhexiline maleate, in robustly suppressing the growth of these organoids.
Dr. Todd Evans, vice chair for research in surgery, study co-senior author and at Weill Cornell Medicine, emphasized the significance of their findings. The research unveiled that the cancer-driving mutation induced abnormal cholesterol production in the organoids, a phenomenon effectively reversed by perhexiline maleate. ” We found that hyperactive cholesterol production is a weak point in many pancreatic cancers, making it a key target for future treatment in most pancreatic cancers,” remarked Dr. Evans.
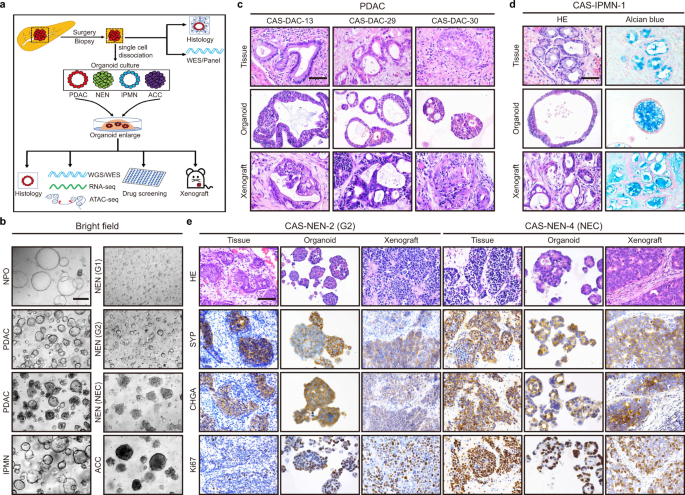

Expanding on this promising avenue, another study conducted by a research team from Japan, published in the Cell Reports journal, tackled the complexity of PDAC’s unique tumor microenvironment (TME). The team, including Dr. Takeuchi Kenta, Professor Taniguchi Hideki, and Associate Professor Tanimizu Naoki, pioneered the creation of pancreatic cancer organoid systems that closely mimic the PDAC TME.
The system, utilizing human-induced pluripotent stem cell (hiPSC)-derived cells, resulted in two distinct fused pancreatic cancer organoids—proliferative (pFPCO) and quiescent (qFPCO)—mirroring the characteristics of patient PDAC tissue.
The significance of this research lies in the ability to replicate the intricate TME involving various cancer-associated fibroblasts (CAFs) within a cell culture model. Dr. Tanimizu Naoki emphasized the potential of their PDAC model for anticancer drug screening, considering the limited treatment options for this challenging disease.
Furthermore, organoids have emerged as key tools in disease and health for studying tissues. Weill Cornell Medicine researchers utilized an organoid-based automated drug-screening system specifically tailored for PDAC, employing normal mouse pancreatic tissue engineered with cancer-driving mutations. Among the compounds tested, perhexiline maleate demonstrated remarkable efficacy, inhibiting the growth of organoids containing the cancer-driving mutation without adverse effects on healthy organoids.
The study revealed that mutant Kras, a common driver of PDAC, in organoid cells significantly increases cholesterol levels. Perhexiline maleate counteracted influence by inhibiting the regulatory factor SREBP2, suggesting that targeting cholesterol synthesis could be a promising strategy against PDAC.
While perhexiline maleate’s potential as a PDAC treatment is hindered by side effects, the researchers are optimistic. Dr. Shuibing Chen, co-senior author and director of the Center for Genomic Health, highlighted the drug’s potential for modification to enhance its properties, paving the way for the development of a refined PDAC drug.
These innovative studies underscore the transformative potential of organoid-based research in unraveling the complexities of pancreatic cancer and identifying targeted therapies that could reshape the landscape of PDAC treatment.